Taking VENCLEXTA
YOUR TREATMENT JOURNEY WITH VENCLEXTA
Taking VENCLEXTA
Find out more about taking VENCLEXTA by watching these three short videos:
Why is treatment with VENCLEXTA tablets only given for 2 years when prescribed with rituximab? (2m 36s)
For further details see Safety Information.
HOW IS VENCLEXTA GIVEN
There are three main phases of treatment that last about two years (24 months) in total - VENCLEXTA alone, VENCLEXTA in combination with rituximab and finally a maintenance period of VENCLEXTA alone

Phase 1 is VENCLEXTA alone (gradual dose-increase phase)
Your doctor will start you on a low dose of VENCLEXTA and gradually increase the amount you take until you are at the full dose. This phase usually lasts about 5 weeks, but your doctor may extend this dose increase period based on your response to the therapy.
This phase of gradually increasing the dose is important because certain side effects are more likely to occur when first starting treatment. But by starting on a low dose, and having your response closely monitored by your doctor as the dose is increased, the risk of these side effects can be reduced.
Blood tests will be done before treatment starts and at each weekly dose increase.
Do not stop using this medicine or change your dose without checking with your doctor.
Tablets should be swallowed whole with a full glass of water without chewing, crushing, or breaking them. Take the tablets during or immediately after a meal, at about the same time every day.
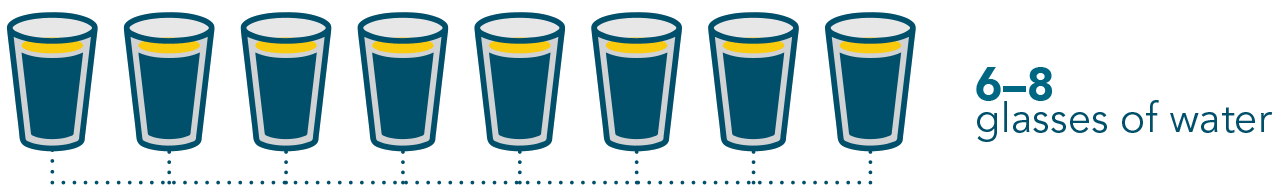
It is also important to stay hydrated during treatment with VENCLEXTA and drink at least 6-8 glasses (1.5 to 2 litres) of water a day, especially starting two days before and on the day of your first dose of VENCLEXTA and every time the dose is increased to reduce the risk of side effects.
Phase 2 is VENCLEXTA with rituximab
Once you’ve completed Phase 1, the rituximab infusions will then begin.
You will continue taking the full dose of your VENCLEXTA treatment throughout this phase, as directed by your doctor. That’s usually four x 100 mg tablets per day, taken together as one dose, with food.
While VENCLEXTA comes as an oral tablet that you can take at home, rituximab is given by your nurse or doctor as an infusion through a needle placed in a vein (intravenous infusion) in your arm.
You will be given rituximab 6 times in total, once every 28 days. This is sometimes referred to as “6 cycles” of rituximab.
Your healthcare team will discuss scheduling these infusion appointments with you and explain how long they will take.
Phase 3 is VENCLEXTA alone, a maintenance phase
After your last rituximab infusion, you will continue taking the full dose of your VENCLEXTA treatment (that’s usually four x 100 mg tablets once daily), for another 18 months unless your doctor tells you to stop or reduce the dose temporarily.
You do not need to keep taking VENCLEXTA forever. Once you reach a full 24 months since your first rituximab infusion, you will be able to stop VENCLEXTA.
It is important to remember to take your tablets every day as directed by your doctor until your doctor tells you to stop.
If your CLL returns, either while taking VENCLEXTA or at some time after you have stopped taking it, your doctor will also discuss your treatment options with you.
WHEN AND HOW TO TAKE VENCLEXTA
Take VENCLEXTA exactly as your doctor has prescribed. If you have questions about how to take VENCLEXTA, ask your doctor or pharmacist.



STAYING HYDRATED
It is important to stay hydrated when taking VENCLEXTA and drink about 6 to 8 glasses (1.5 to 2 litres) of water each day as instructed.
Make especially sure you drink your 6-8 glasses of water on these days:
- The two days before, and the day that you take your first VENCLEXTA dose
- The two days before, and the day you increase your VENCLEXTA dose.
IF YOU MISS A DOSE OF VENCLEXTA
It is important not to miss a dose of this medicine.
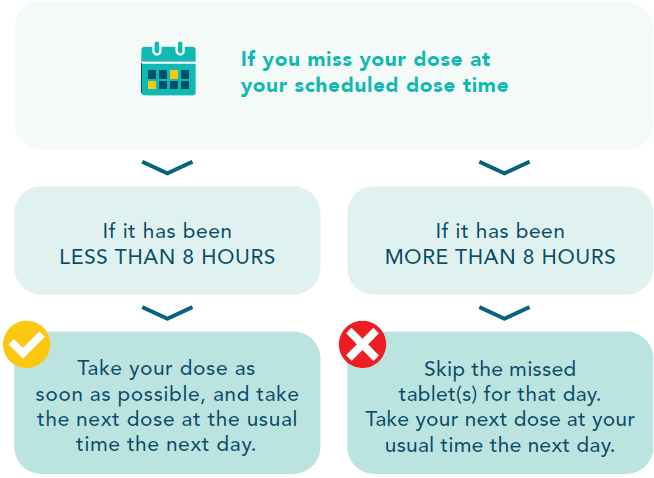
If you vomit after taking VENCLEXTA, do not take an extra dose. Take the correct dose at your usual time the next day. If you are unsure, talk to your doctor, nurse or pharmacist.
If you think you (or anyone else) may have taken too much VENCLEXTA, immediately telephone your doctor or the National Poisons Centre on 0800 764 766 or go to Accident and Emergency at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning.
QUESTIONS TO ASK YOUR DOCTOR ABOUT VENCLEXTA
You may have many questions for your doctor about VENCLEXTA and sometimes it can be hard to remember everything. Writing down the questions in advance can help.
Here are some questions to get you started.
- What is the medicine?
- Can I take it from home or in a clinic?
- Are there any special dietary requirements when taking the medicine?
- How long will I need to take it for?
- How will it make me feel?
- What can you tell me about any side effects with this medicine?
- How will we know if it is working?
- What can I do to reduce or manage side effects?
- When should I start taking the medicine?
- What other options are available to me?
- Who will I contact if I have any questions?
NZ-VEN-240013. TAPS BG4140. July 2024.
For more information about AbbVie, the maker of VENCLEXTA, visit www.AbbVie.co.nz
For medical information enquiries regarding VENCLEXTA please contact [email protected]
The content on this site is intended solely for New Zealand residents. The information is intended for informational purposes only and should not be used to replace a discussion with a healthcare professional. All decisions made regarding patient care must be handled by a healthcare professional and be made based on the unique needs of each patient. This website has been developed and is funded by AbbVie Limited, Wellington. The person shown is from stock photography (model) and is not an actual patient.
VENCLEXTA in combination with rituximab is fully funded for relapsed/refractory chronic lymphocytic leukaemia (CLL). Special Authority criteria apply. Refer to the VENCLEXTA PHARMAC Special authority criteria. VENCLEXTA in combination with obinutuzumab or ibrutinib for 1L CLL is not funded - a charge will apply. VENCLEXTA is not funded for acute myeloid leukaemia (AML) - a charge will apply.
IMPORTANT INFORMATION ABOUT VENCLEXTA®
VENCLEXTA® is a prescription medicine containing venetoclax. Venclexta is available as film-coated tablets of various strength (10 mg, 50 mg, 100 mg venetoclax). It is used to treat adults with chronic lymphocytic leukaemia (CLL) or small lymphocytic lymphoma (SLL). Venclexta is taken alone or in combination with other medicines. Your doctor will let you know which combination medicines, how to take them, and how long to take them. Typically, you will start treatment with Venclexta at a low dose. If you are taking Venclexta for CLL or SLL, your doctor will gradually increase your dose over 5 weeks up to the full dose. Use strictly as directed by your doctor. Venclexta has risks and benefits. You must not take it if you are allergic to venetoclax or to any of the inactive ingredients. For CLL or SLL, do not take Venclexta if you are taking any of the following medicines: medicines used to treat or prevent fungal infections, including ketoconazole, posaconazole, voriconazole, itraconazole; clarithromycin (an antibiotic); or ritonavir (a medicine used to treat HIV and hepatitis C). Do not drink grapefruit juice, or eat grapefruit, starfruit or Seville oranges or marmalades. Do not give Venclexta to children and adolescents under 18 years of age. Do not take Venclexta if you are pregnant or plan to become pregnant, or if you are breastfeeding or plan to breastfeed. If you are a woman of child-bearing age, you must use a highly effective form of contraception during treatment with Venclexta and for at least 30 days after your last dose. Tell your doctor, nurse, or pharmacist if you have any kidney or liver problems; if you think you may have an infection; or if you recently received or are scheduled for any vaccinations. Do not stop using Venclexta or change the dose without checking with your doctor. Venclexta can cause tumour lysis syndrome (TLS) , which is caused by the fast breakdown of cancer cells. TLS is a very serious side effect that can be fatal. TLS is most likely to occur when you are first starting treatment. To help prevent TLS, it is important to stay hydrated and drink water every day when taking Venclexta. Particularly, starting two days before and on the day of your first dose of Venclexta and every time the dose is increased, drink 6 to 8 glasses (approximately 1.5-2 L total) of water each day. Let your healthcare provider know immediately if you experience: fever or chills; feeling sick or vomiting; being short of breath; feeling unusually tired; changes in your heart rate (slow, fast or irregular); your urine looks dark or cloudy; feeling confused; convulsions or fits; or pain in the muscles or joints while on treatment with Venclexta. Ensure you follow all your doctor’s instructions carefully and keep all your appointments, including those for blood tests. You may experience a low number of neutrophils, a type of white blood cells – this can be severe and need treatment. Your doctor will check your blood counts during treatment with Venclexta. You may experience infections during treatment with Venclexta. Some infections can be very serious or even fatal. Your doctor will closely monitor and treat you right away if you have fever or any signs of infection during treatment with Venclexta. Tell your doctor immediately if you have signs of an infection before, or while taking Venclexta, including: fever or chills, feeling weak or confused, cough, runny nose, sore throat; congestion on the chest; or pain or burning when passing urine. Some of the less serious side effects of Venclexta include diarrhoea; tummy pain; constipation, nausea (feeling sick); vomiting; reduced appetite; weight loss; mouth sores; looking pale; feeling tired; having little or no energy; shortness of breath when exercising; feeling dizzy; headache; low blood pressure; bleeding. Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Some medicines and Venclexta may interfere with each other, so tell your doctor if you are taking medicines containing any of the following: fluconazole, ciprofloxacin, erythromycin, diltiazem, verapamil, captopril, felodipine, dronedarone, amiodarone, quinidine, rifampicin, carvedilol, ciclosporin, quercetin, ranolazine, ticagrelor, azithromycin, nafcillin, carbamazepine, phenytoin, St John’s wort (Hypericum perforatum), bosentan, efavirenz, etravirine, modafinil, warfarin, digoxin, everolimus, or sirolimus. Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without prescription. Tell any other doctors, pharmacists, dentists, or surgeons treating you that you are taking Venclexta and remind them before you start any new medicines. If you have any questions about using Venclexta, including its risks and benefits, how much to use, how and when to use it, or storage conditions, ask your healthcare professional and refer to the Consumer Medicine Information (CMI) available from www.medsafe.govt.nz or free phone 0800 900 030. Ask your doctor if Venclexta is right for you. Use strictly as directed. If symptoms continue, or you have side effects, see your doctor, pharmacist, or healthcare professional. V7a.
©2024 AbbVie. All rights reserved. AbbVie® is a registered trademark of AbbVie Inc. VENCLEXTA® is a registered trademark of AbbVie Manufacturing Management Unlimited Company. AbbVie Limited, PO Box 11437, Manners Street, Wellington 6142. TAPS BG4140. NZ-VEN-200006. ONO0142. September 2024.

